
(2020.V1)NCCN临床实践指南:骨髓增殖性肿瘤——原发性血小板增多症
指南简介
Introduction to the guide
美国国家综合癌症网络(NCCN)发布了骨髓增殖性肿瘤管理指南2020年第1版,指南主要内容涉及骨髓增殖性肿瘤,骨髓纤维化,真性红细胞增多症以及原发性血小板增多症的诊断,检查以及治疗的相关内容。
01
推荐意见 (译文)
骨髓纤维化(MF)、真性红细胞增多症(PV)和原发性红细胞增多症血小板增多症(ET)是一类造血系统异常的疾病统称为骨髓增生性肿瘤(MPN)。在美国,现存的MF、ET和PV患者人数大约分别为13000、134000和148000,在最近的一项调查中,美国(2001-2012)MPN的不同亚类的发病率(IRs)有所差异,其中PV的发病率(IRs=11)最高,ET发病率次之(IRs=10)。
MPN具有复杂的临床表征,不同亚类的MPN临床特征也相应的有所不同,但是大多类似,例如疲劳,瘙痒,体重减轻,脾肿大以及实验室检测项目的一些异常,包括红细胞增多,血小板增多,白细胞增多。SEER-Medicare数据库分析显示MF患者的生存期是明显低于ET患者或PV患者的。此外,MPN患者有几率会转为加速期以及急性髓性白血病(AML)。
自从JAK2、CALR和MPL相关基因突变与疾病的相关性被发现以及靶向治疗技术的新兴和发展,MPN的诊断和治疗水平也在不断提升,对于缓解患者症状以及提高生存质量有十分重要的意义。然而,根据诊断评估患者疾病症状负担以及选择合适的治疗方案仍然是一个不小的挑战。本指南将根据诊断检查、风险分级、治疗方案等几个方面提供建议。本篇将重点介绍原发性血小板增多症(ET)的相关治疗方案。
02
推荐意见 (译文)
1 基于危险分层的治疗建议
1.1 极低危原发性血小板增多症(ET)患者(年龄≤60岁,没有JAK2突变,也没有血栓史)和低危患者(年龄≤60岁,存在JAK2突变,没有血栓史),采用观察随诊策略,监测是否有新的血栓形成,血管性血友病,和/或大出血;81-100 mg/d的阿司匹林的剂量可减少血栓等血管症状的风险。
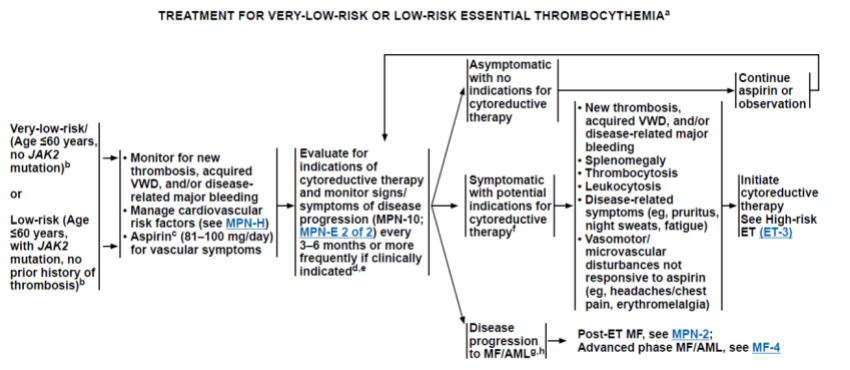
Fig.1 极低危、低危ET患者治疗路线
1.2 中危ET患者(年龄>60岁,没有JAK2突变,没有血栓史)监测是否有新的血栓形成,血管性血友病,和/或疾病相关的大出血;心血管相关的危险因素;81-100 mg/d的阿司匹林的剂量可减少血栓等血管症状的风险
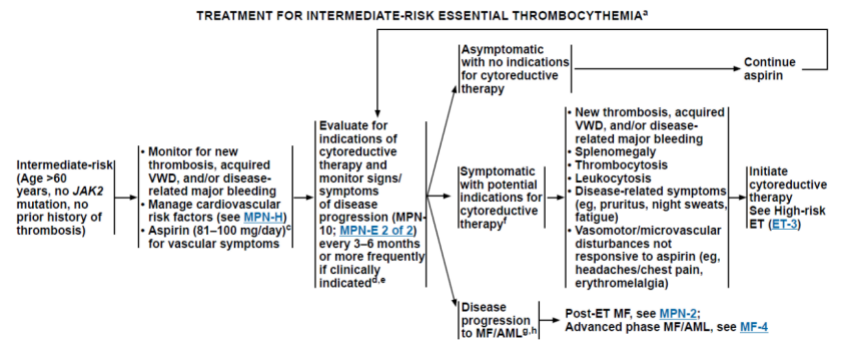
Fig.2 中危ET患者治疗路线
1.3 高危ET患者(有血栓史的任何年龄患者,或者年龄>60岁并伴有JAK2基因突变的患者),除监测心血管事件、新发生的血栓和出血情况外应采取降细胞治疗。
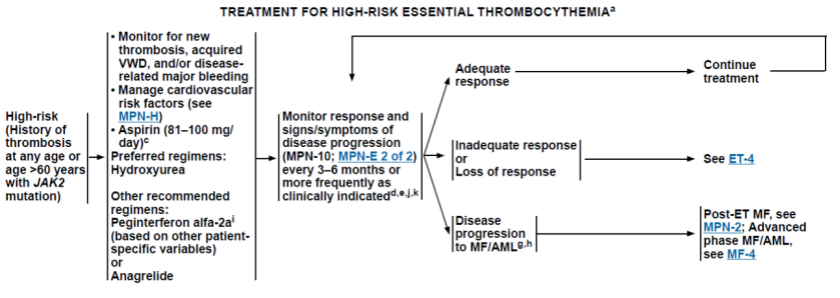
Fig.3 高危ET患者治疗路
初始治疗患者的降细胞治疗药物包括羟基脲和阿那格雷联合81-100 mg/d的阿司匹林、干扰素α。
低剂量的阿司匹林在ET患者中治疗的安全性以及有效性仍然未在随机临床实验中得到评估,最近的一篇系统性综述也表明阿司匹林在ET患者的抗血小板治疗中的安全性与有效性仍然是高度不确定的。但是阿司匹林(81-100 mg/d)依然可以考虑在极低危、低危以及中危ET患者中使用,用以降低血栓等并发症的风险。
最近的一份回顾性分析报告表明,阿司匹林的使用对于CALR突变的ET患者并不是有利的,在对多名患者的研究中发现,低剂量的阿司匹林对于降低血栓的形成并无影响却与较高的出血率有关,但是该研究仍然缺乏更进一步的研究证实。因此目前专家组认为没有足够的证据停止阿司匹林在CALR突变的ET患者中的使用。
年轻患者推荐使用干扰素α或者聚乙二醇干扰素。年龄较小的患者、需要降细胞治疗的孕妇更适合选用干扰素进行降细胞治疗,不可使用羟基脲。
参考译文:
Very-low-risk (age ≤60 years without JAK2 mutation and no prior history of thrombosis) or Low-risk (age ≤60 years with JAK2 mutation and no prior history) or Intermediate-risk (age ˃60 years, no JAK2 mutation, and no prior history of thrombosis)
As discussed above, the efficacy and safety of low-dose aspirin in patients with ET has not been evaluated in randomized clinical trials. The results of a recent systematic review also suggest that the risks and benefit of antiplatelet therapy in patients with ET remains highly uncertain. Observation is appropriate for patients with very-low-risk or low-risk ET.
Aspirin (81–100 mg/d) could be considered to reduce the risk of thrombotic complications for patients with very-low-risk, low-risk, or intermediate-risk ET. Aspirin should be used with caution in patients with acquired VWD who have an increased risk of bleeding.
A report from a more recent retrospective analysis suggests that the use of low-dose aspirin may not be beneficial in patients with low-risk CALR-mutated ET. In an analysis that evaluated the benefit-to-risk ratio of low-dose aspirin in 433 patients with low-risk ET (271 patients with CALR mutation and 162 patients with a JAK2 V617F mutation) who were on antiplatelet therapy or observation, low-dose aspirin did not affect the risk of thrombosis but was associated with a higher incidence of bleeding in patients with CALR-mutated ET. These findings have to be confirmed in prospective clinical trials. Therefore, at present, the panel feels that there is not enough evidence to recommend withholding aspirin for patients with CALR-mutated ET.
In carefully selected patients, twice-daily aspirin at a 100-mg dose has been found to be more effective than once-daily aspirin (100 mg), a finding that has yet to be confirmed in randomized controlled studies. The safety and efficacy of different aspirin dosing regimens in patients with ET is being evaluated in a phase II randomized trial. At the present time, the risk and benefits of higher dose aspirin must be weighed based on the presence of vasomotor symptoms and the risk of bleeding. It may be appropriate in carefully selected patients as clinically indicated. High-risk (History of thrombosis at any age or >60 years with JAK2 mutation) Cytoreductive therapy (hydroxyurea or anagrelide) with aspirin (81–100 mg/d) is recommended as initial treatment. Interferon alfa-2b, peginterferon alfa-2a, or peginterferon alfa-2b could be considered for younger patients, in pregnant patients requiring cytoreductive therapy, or in those patients requiring cytoreductive therapy that defer hydroxyurea.
